Although Helicobacter pylori infection is both a common and a serious bacterial infection, antimicrobial therapies have rarely been optimized, are prescribed empirically, and provide inferior results compared with antimicrobial therapies for other common infectious diseases. The effectiveness of many of the frequently recommended H. pylori infection treatment regimens has been increasingly compromised by antimicrobial resistance. Regional data on the susceptibility of strains of H. pylori to available antimicrobials are sorely needed. Noninvasive molecular methods are possible to assess clarithromycin susceptibility in isolates obtained from stool specimens. As a general rule, clinicians should prescribe therapeutic regimens that have a ≥90% or, preferably, ≥95% eradication rate locally. If no available regimen can achieve a ≥90% eradication rate, clinicians should use the most effective regimen(s) available locally. Eradication of infection should always be confirmed after treatment in order to provide feedback regarding local effectiveness and an early warning of increasing resistance. In most regions of the world, four-drug treatment regimens, including a PPI plus three antimicrobials (clarithromycin, metronidazole/tinidazole and amoxicillin), or a PPI plus a bismuth plus tetracycline and metronidazole provide the best results. Standard triple therapy (a PPI, amoxicillin and clarithromycin) should now be avoided owing to increasing resistance to this treatment.
Choice of Therapy
Despite the availability of various methods of susceptibility testing, as mentioned earlier, susceptibility testing is rarely performed and most patients with H. pylori infection receive an empirically chosen treatment. Clinicians are advised to use what works best locally and always confirm cure with a post-treatment, preferably non-invasive, test.[14] Monitoring the outcome of therapy provides clinicians with a reliable measure of the local effectiveness of a particular therapy and also serves as an early warning of development of resistance, which may undermine the effectiveness of a particular regimen.
Choice of therapy can be improved by knowledge of the local or regional patterns of drug resistance and the patient's history of prior antibiotic use, which can assist in identifying drugs to which resistance is likely to be present (for example, previous use of macrolides, fluoroquinolones, or metronidazole). Fluoroquinolone resistance is rapidly increasing;[32,65,66]therefore, the finding of previous use of this drug should discourage reuse without susceptibility testing. With few exceptions worldwide, the prevalence of clarithromycin resistance has increased to a level where standard triple therapy is no longer effective and clinicians should therefore avoid triple therapies (Figure 2) .[14] Figure 3 depicts the theoretical effect of increasing clarithromycin resistance on per protocol treatment success with standard triple therapy for 7 or 14 days. Treatment success falls below 90% when resistance is >5% with 7-day therapy, and >10% with 14-day therapy.
Figure 2:
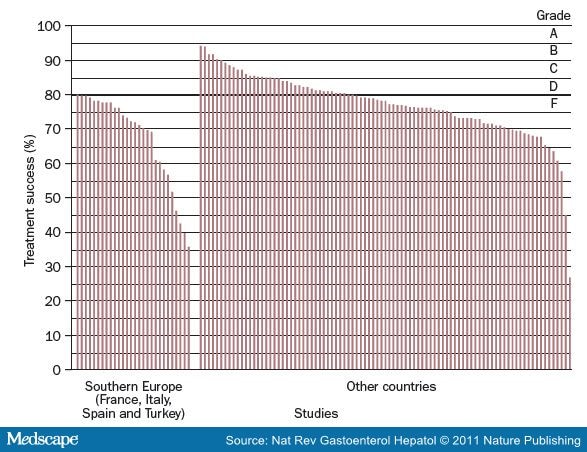
Percentage of Treatment Success for Triple Therapy in Studies of Populations in Southern Europe and Other Geographic Areas According to Intention-to-treat Analysis. Triple therapy contains a PPI, clarithromycin, and amoxicillin. The line at 80% treatment success defines the demarcation between acceptable and unacceptably low treatment success. Grades A, B, C, D, and F on the right-hand side of the graph refer to ordered categories of grading Helicobacter pylori infection treatment success (e.g. A = >95% or greater) for intention-to-treat analysis.86 Reproduced from Graham et al. Gut 59, 1143-1153 © 2010 with permission from BMJ publishing group.
Figure 3:
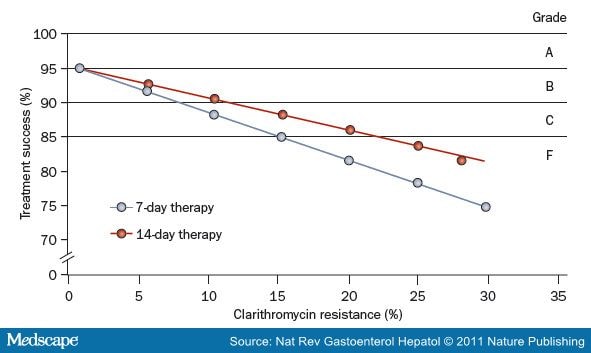
The Theoretical Effect of Increasing Clarithromycin Resistance on the Success of Treatment ofHelicobacter pylori Infection with Triple Therapy (a PPI, Clarithromycin and Amoxicillin) for 7 or 14 Days. Data are based on a 95% treatment success rate for susceptible strains and a 50% or 28% success rate for resistant strains treated for 7 days or 14 days, respectively. As shown, treatment success falls below 90% with 10% clarithromycin resistance for 7-day therapy and 15% resistance for 14-day therapy according to per protocol analysis.86 Grades A, B, C, and F on the right-hand side of the graph refer to ordered categories of grading H. pylori infection treatment success (e.g. A=>95% or greater) for per protocol analysis.
Standard triple therapy only remains a good treatment choice when the infection is known to be susceptible to clarithromycin. When antimicrobial resistance is not known, clinicians should use a regimen that, based on current evidence, is optimal in terms of efficacy, dose, duration, dosing interval and local antimicrobial resistance (Table 2 ).
Table 2. Recommended Regimens for Helicobacter pylori Therapy—2011 to 2012
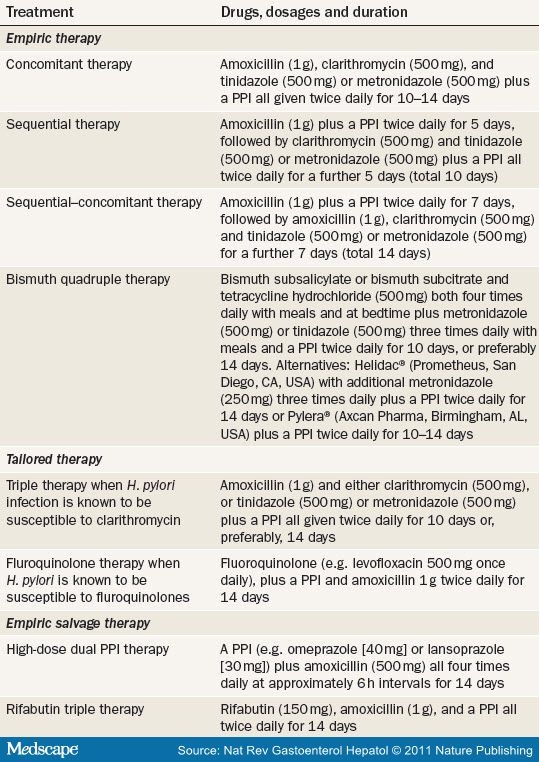
Recommended Empiric Regimens
Sequential therapy and concomitant therapy are both nonbismuth-containing four-drug regimens comprised of a PPI, clarithromycin, metronidazole and amoxicillin. Sequential therapy is administered in two parts: a PPI plus amoxicillin for 5 days followed by a PPI plus clarithromycin and metronidazole for an additional 5 days. In concomitant therapy, all four drugs are administered for the entire duration of therapy and this regimen is therefore less complex than sequential therapy as it does not involve changing the number or type of drugs halfway through. These regimens are generally effective except in areas with a high prevalence (>20-30%) of clarithromycin resistance, or dual clarithromycin and metronidazole resistance.[67-69] Both therapies usually provide a success rate of 90-94%, but neither has been optimized, for example in terms of dose or duration.[70] A 2010 study examined the effect of extending the duration of sequential therapy to 14 days (7 days plus 7 days instead of the usual 5 days plus 5 days) but this did not improve treatment success.[71] Another study examined a sequential-concomitant hybrid therapy, which involved extending the duration of sequential therapy to 14 days and continuing amoxicillin throughout the entire duration of therapy.[71] In 117 individuals, the regimen provided 99% treatment success in per protocol analysis and 97% treatment success in intention-to-treat analysis.[71] Additional studies in populations with different levels of prevalence of clarithromycin and metronidazole resistance are needed to confirm this finding.
Bismuth-containing quadruple therapies are an alternative to quadruple regimens containing clarithromycin.[14,72]During the initial development of bismuth quadruple therapy it was shown that dose and duration of bismuth therapy were important variables, especially in regions where metronidazole resistance was common.[18]However, many of the studies of such regimens have used suboptimal doses and durations of bismuth therapy and, as expected, have achieved relatively poor results.[73] A regimen involving the administration four times daily of three capsules containing a combination of bismuth subcitrate (420 mg), metronidazole (375 mg), and tetracycline (375 mg) plus omeprazole (20 mg) given twice daily has been introduced in the US and is under regulatory review in Europe. Three studies each with more than 100 patients have reported treatment success rates of >90% with this combination given for 10 days.[74-76] The success of this therapy for the treatment of metronidazole-resistant H. pylori strains was reported to be 90% in the US and 90.5% in Europe according to per protocol analysis.[75,76] However, in the European study metronidazole resistance was assessed by use of the Etest®, which, as stated previously, tends to overestimate the prevalence of metronidazole resistance.[16] An eradication rate of 92% has been achieved in patients with metronidazole-resistant H. pylori infection by use of a 14-day, full-dose regimen containing tetracycline (500 mg four times daily), metronidazole (500 mg three times daily), bismuth subsalicylate (two tablets four times daily) and omeprazole (20 mg once daily).[77] As noted previously, under conditions of antibiotic resistance, dose and duration of therapy seem to be important variables with regard to treatment outcome.
An optimal therapeutic regimen for H. pylori infection should produce a >95% rate of treatment success in patients infected with susceptible strains and should still achieve a high eradication rate (that is, >85%) in patients infected with antimicrobial-resistant strains. Bismuth-containing quadruple therapies have not yet been optimized and comparative studies are sorely needed to identify the optimum regimen for this therapy in terms of dosing and frequency of bismuth, tetracycline, metronidazole, and PPI administration. In addition, comparisons of bismuth subsalicylate and bismuth subcitrate remain to be performed. Bismuth quadruple therapy has been underutilized in clinical practice and it is a good choice for first-line therapy for H. pylori infection. In the US, where generic bismuth subsalicylate, metronidazole, tetracycline and PPIs are all available, the cost of a full-dose 14-day course of bismuth-containing quadruple therapy is less than us$50, making it not only highly effective but also cost effective. First-line therapy is usually a choice between a clarithromycin-containing, nonbismuth-containing, four-drug regimen and bismuth quadruple therapy ( Table 2 ).
Second-line Therapy
Second-line therapy is given after the failure of one treatment attempt using first-line therapy. Unless susceptibility testing is available, clinicians can assume that the infection is resistant to clarithromycin and fluoro-quinolone if either of these antibiotics have been used previously or were included as part of the initial regimen. The best advice is to use antimicrobials that were not used as part of the initial treatment regimen. Bismuth, tetracycline, metronidazole and amoxicillin can all be used as part of second-line therapy. In general, the full recommended doses of drugs and duration of treatment should be used in second-line therapy. The preferred approach for second-line therapy is to choose a therapy that has not been used previously.
Conclusions
Although H. pylori infection is both a common and a serious bacterial infection, current antimicrobial therapies have rarely been optimized, are prescribed empirically, and provide inferior results compared with antimicrobial therapies for other common infectious diseases. In most regions of the world, four-drug treatment regimens including a PPI and three antimicrobials provide the best results; triple therapy with a PPI, amoxicillin and clarithromycin should be avoided ( Table 2 ).
Key Points
- New treatment regimens for Helicobacter pylori infection should be optimized to achieve an eradication rate of >95% before their use in clinical practice
- A patient's allergies and the local availability of drugs should be considered when selecting a treatment
- If there is no available therapy with an eradication rate ≥90%, the most locally effective therapy available should be employed
- Treatment should involve high drug doses (for example, 500 mg of clarithromycin, metronidazole or tetracycline) for 14 days unless lower doses and a shorter duration of therapy are equally effective locally
- H. pylori eradication should be confirmed after treatment, and, if second-line therapy is required, clarithromycin or a fluoroquinolone should not be reused
- Salvage therapy (after multiple treatment failures) should be chosen on the basis of susceptibility testing whenever possible
References
- Kandulski, A. et al. Helicobacter pylori infection: a clinical overview. Dig. Liver Dis. 40, 619-626 (2008).
- Sarai, A. S. et al. Helicobacter pylori, a causative agent of vitamin B12 deficiency. J. Infect. Dev. Ctries 2, 346-349 (2008).
- Shiotani, A. & Graham, D. Y. Pathogenesis and therapy of gastric and duodenal ulcer disease. Med. Clin. North Am. 86, 1447-1466 (2002).
- Graham, D. Y. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology 115, 1272-1277 (1998).
- Graham, D. Y. & Shiotani, A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat. Clin. Pract. Gastroenterol. Hepatol. 5, 331 (2008).
- Megraud, F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 53, 1374-1384 (2004).
- Megraud, F. Helicobacter pylori and antibiotic resistance. Gut 56, 1502 (2007).
- Gisbert, J. I? The recurrence of Helicobacter pylori infection: incidence and variables influencing it. A critical review. Am. J. Gastroenterol. 100, 2083-2099 (2005).
- Graham, D. Y. Helicobacter pylori eradication therapy research: ethical issues and description of results. Clin. Gastroenterol. Hepatol. (in press).
- Fischbach, L. A., Goodman, K. J., Feldman, M. & Aragaki, C. Sources of variation of Helicobacter pyloritreatment success in adults worldwide: a meta-analysis. Int. J. Epidemiol. 31, 128-139 (2002).
- Borody, T. J. et al. Omeprazole enhances efficacy of triple therapy in eradicating Helicobacter pylori. Gut 37,477-481 (1995).
- Borody, T. J. et al. Optimal H. pylori (HP) therapy—a combination of omeprazole and triple therapy (TT).Gastroenterology 106, A55 (1994).
- de Boer, W. A. How to achieve a near 100% cure rate for H. pylori infection in peptic ulcer patients. A personal viewpoint. J. Clin. Gastroenterol. 22, 313-316 (1996).
- Graham, D. Y. & Fischbach, L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut59, 1143-1153 (2010).
- Graham, D. Y. et al. Practical rapid, minimally invasive, reliable nonendoscopic method to obtain Helicobacter pylori for culture. Helicobacter 10, 1-3 (2005).
- Osato, M. S. et al. Comparison of the Etest and the NCCLS-approved agar dilution method to detect metronidazole and clarithromycin resistant Helicobacter pylori. Int. J. Antimicrob. Agents 17, 39-44 (2001).
- Osato, M. S. et al. Pattern of primary resistance of Helicobacter pylori to metronidazole or clarithromycin in the United States. Arch. Intern. Med. 161, 1217-1220 (2001).
- van der Wouden, E. J. et al. The influence of in vitro nitroimidazole resistance on the efficacy of nitroimidazole-containing anti-Helicobacter pylori regimens: a meta- analysis. Am. J. Gastroenterol.94, 1751-1759 (1999).
- Graham, D. Y. et al. Sequential therapy using high-dose esomeprazole-amoxicillin followed by gatifloxacin forHelicobacter pylori infection. Aliment. Pharmacol. Ther. 24, 845-850 (2006).
- Dore, M. P et al. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome ofHelicobacter pylori therapy: a meta-analytical approach. Dig. Dis. Sci. 45, 68-76 (2000).
- Bardhan, K. et al. The HOMER Study: the effect of increasing the dose of metronidazole when given with omeprazole and amoxicillin to cure Helicobacter pylori infection. Helicobacter 5, 196-201 (2000).
- Versalovic, J. et al. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 40, 477-480 (1996).
- Taylor, D. E. et al. Cloning and sequence analysis of two copies of a 23S rRNA gene from Helicobacter pyloriand association of clarithromycin resistance with 23S rRNA mutations. Antimicrob. Agents Chemother. 41,2621-2628 (1997).
- Versalovic, J. et al. Point mutations in the 23S rRNA gene of Helicobacter pylori associated with different levels of clarithromycin resistance. J. Antimicrob. Chemother. 40, 283-286 (1997).
source: www.medscape.com